

News
Bio-tech firm develops 3D printed replacement cornea for human eyes
After successfully transplanting the first 3D-printed cornea in an animal, North Carolina company Precise Bio has recently announced the launch of a dedicated business for creating marketable, 3D-printed products for human eyes. Founded by scientists from the Wake Forest Institute of Regenerative Medicine, this company is developing bio-fabrication printers that can restore cells, tissues, and organs. Their proprietary technology, a 4D bio-printing platform, is said to resolve existing limitations presented by other bioprinters to enable more complex tissues to be engineered for transplants and treatments. By focusing on developing marketable products for the eye, the company aims to achieve rapid advancement in its field and move to overhaul the whole organ transplant system.
A cornea transplant with sutures still visible. | Credit: National Institutes for Health, National Eye Institute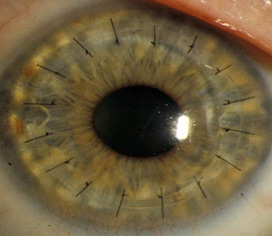
When a cornea is damaged by disease or injury, a replacement is often needed to restore vision. Transplant surgery using donated corneas is an available solution, however, it relies on a deceased donor. While the waiting list in the United States is nearly non-existent, other countries require longer wait times, some over a year, before one is available. The Eye Bank Association of America estimates that around 10 million people suffer from corneal blindness that could potentially be restored via transplant surgery. An artificially manufactured cornea would overcome supply limitations while also contributing to the knowledge base to develop more complex organs such as hearts and livers.
The cornea is the transparent layer covering the front part of the eye that, along with the lens, accounts for about two-thirds of the eye’s optical power. It does not contain blood vessels, making it a prime candidate for bioprinting, the field of 3D printing involving biological materials. Bioprinters differ from traditional 3D printers in ways that might not be surprising given their name. Instead of heated filament applied in layers on a plate to build an object, layers of cells and biocompatible materials are printed to form tissue. Along with a lack of blood vessels, the layered structure of the cornea also makes it well suited for bioprinting.
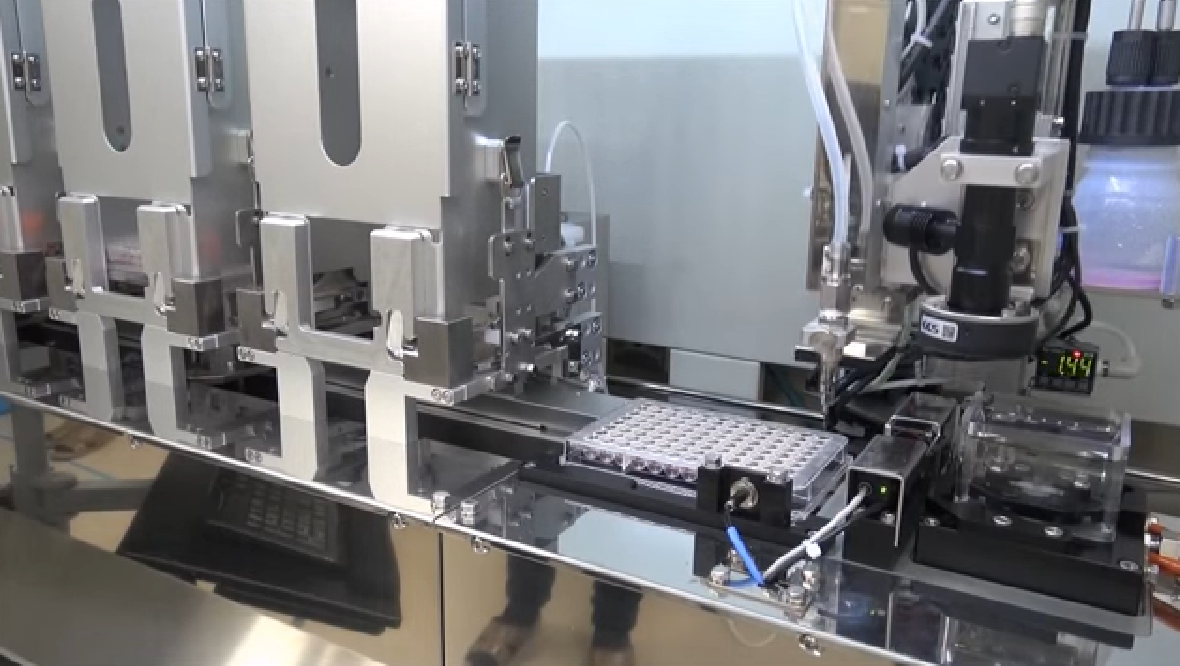
Precise Bio refers to its printing technique used for the corneas and other biomaterials as “4D” over the usual “3D” label due to the curing stage for the printed cells. The fourth dimension referred to is time needed, 10-14 days specifically, for the bio-printed cells and fibers to connect themselves together for biological operation in a bioreactor that keeps them warm for the duration. Aryeh Batt, Precise Bio’s CEO, summarized this step in an interview with IEEE Spectrum: “Essentially, the biology does the work, but you have to put them in the correct environment to make it happen.”
Initial animal safety studies for the corneal transplant have already been completed, and now the company must clear some additional hurdles to begin human testing. One of the major proofs for Precise Bio will be the demonstration of normal behavior of the bio-printed products. For example, during production using the 3D/4D printer, the printed cells grow rapidly into the form needed in a way that does not occur in a normal eye. The company will have to demonstrate in clinical studies how this process is controlled and stopped before transplant.
The field of 3D bioprinting is a research area receiving a significant amount of attention due to its potential for developments in tissue engineering, drug delivery, and cancer studies. In fact, hundreds of scientific articles were published on the topic this year alone. As with most new technology, however, the challenge of moving a development out of the laboratory and into the consumer marketplace is significant, but Precise Bio hopes to meet it head on with its new dedicated business.
For more on bioprinting, watch the below Tedx Talk by Dr. Sam Wadsworth of Aspect biosystems Ltd.

Elon Musk
Tesla is ramping up its advertising strategy on social media
Tesla has long stood out in the automotive world for its unconventional approach to advertising—or, more accurately, its near-total avoidance of it. For over a decade, the company spent virtually nothing on traditional marketing.

Tesla seems to be ramping up its advertising strategy on social media once again. Marketing and advertising have not been a major focus of Tesla’s, something that has brought some criticism to the company from its fans.
However, the company looks to be making adjustments to that narrative, as it has at times in the past, as ads were spotted on several different platforms over the past few days.
On Facebook and YouTube, ads were spotted that were evidently placed by Tesla. On Facebook, Tesla was advertising Full Self-Driving, and on YouTube, an ad for its Energy Division was spotted:
Tesla also threw up some ads on YouTube for Energy https://t.co/19DGQMjBsA pic.twitter.com/XQRfgaDKxY
— TESLARATI (@Teslarati) March 9, 2026
Tesla has long stood out in the automotive world for its unconventional approach to advertising—or, more accurately, its near-total avoidance of it. For over a decade, the company spent virtually nothing on traditional marketing.
In 2022, Tesla’s U.S. ad spend was roughly $152,000, a rounding error compared to General Motors’ $3.6 billion the following year.
Traditional automakers averaged about $495 per vehicle on ads; Tesla spent $0. CEOElon Musk’s stance was explicit: “Tesla does not advertise or pay for endorsements,” he posted on X in 2019. “Instead, we use that money to make the product great.”
The strategy relied on word-of-mouth from delighted owners, Elon’s massive X following, viral product launches, media frenzy, and customer referrals. A great product, Musk argued, sells itself. It does not need Super Bowl spots or billboards. Resources poured into R&D instead, with Tesla investing nearly $3,000 per car, far more than rivals.
Tesla counters jab at lack of advertising with perfect response
This reluctance wasn’t arrogance; it was philosophy, and Musk made it clear that the money was better spent on the product. Heavy spending on ads was seen as wasteful when innovation and authenticity drove organic demand. Shareholder calls for marketing budgets were ignored.
The current shift, paid Facebook ads promoting Full Self-Driving (Supervised) and YouTube Shorts offering up to $1,000 back on Powerwall batteries, marks a pragmatic evolution.
These targeted campaigns coincide with the end of one-time FSD purchases and a March 31 deadline for FSD transfer eligibility on new vehicles.
This move likely signals Tesla adapting to scale, as well as a more concerted effort to stop misinformation regarding its platform. As EV competition intensifies and the company bets big on robotaxis and energy storage, pure organic buzz may not suffice to hit adoption targets. Selective digital ads allow precise, cost-effective reach without abandoning core principles.
If successful, it could foreshadow measured expansion into marketing, boosting high-margin software and home energy revenue while preserving Tesla’s innovative edge. But, it’s nice to see the strategy return, especially as Tesla has been reluctant to change its mind in the past.
News
Tesla Model Y outsells everything in three states, but Ford dominates
The Model Y’s success here highlights accelerating mainstream adoption of electric SUVs, which offer spacious interiors, impressive range, rapid acceleration, and low operating costs.

The Tesla Model Y was the best-selling vehicle in three different states in the U.S. last year, according to new data that shows the all-electric crossover outsold every other car in a few places. However, Ford widely dominated the sales figures with its popular F-Series of pickups.
According to new vehicle registration data compiled by Edmunds and visualized by Visual Capitalist, the Ford F-Series, encompassing models like the F-150, F-250, F-350, and F-450, claimed the title of best-selling vehicle in 29 states.
This dominance underscores the pickup truck’s unbreakable appeal across much of the country, particularly in rural, Midwestern, Southern, and Western states, where towing capacity, durability, and utility for work or recreation remain top priorities.
The Tesla Model Y is the best-selling vehicle in California, Washington, and Nevada
How many states will it dominate next year? https://t.co/ERyoyce42D
— TESLARATI (@Teslarati) March 9, 2026
The F-Series has held the crown as America’s overall best-selling vehicle for decades, a streak that continued strong into 2025 despite broader market shifts.
Yet, amid this truck-heavy reality, Tesla made a notable breakthrough. The Model Y emerged as the top-selling vehicle, not just the leading EV, but the outright best-seller in three key states: California, Nevada, and Washington.
These West Coast strongholds reflect regions with robust EV infrastructure, high environmental awareness, generous incentives, and tech-savvy populations. In California alone, nearly 50 percent of new vehicle registrations were electrified, far outpacing the national average of around 25 percent.
The Model Y’s success here highlights accelerating mainstream adoption of electric SUVs, which offer spacious interiors, impressive range, rapid acceleration, and low operating costs.
Elon Musk: Tesla Model Y is world’s best-selling car for 3rd year in a row
Elsewhere, Japanese crossovers filled many gaps: Toyota’s RAV4 and Honda’s CR-V topped charts in several urban and densely populated Northeastern and Midwestern states, where fuel efficiency, reliability, and family-friendly features win out over larger trucks.
While Ford’s broad reach shows traditional preferences persist, at least for now, Tesla’s Model Y victories in high-population, influential states signal a gradual but undeniable transition toward electrification. As charging networks expand and battery technology improves, more states could follow the West Coast’s lead in the coming years.
This 2025 map captures a pivotal moment: pickup trucks still rule the majority, but EVs are carving out meaningful territory where consumer priorities align with sustainability and innovation. The road ahead promises continued competition between legacy giants and electric disruptors.
Elon Musk
Elon Musk shares updated Starship V3 maiden launch target date
The comment was posted on Musk’s official account on social media platform X.

SpaceX CEO Elon Musk shared a brief Starship V3 update in a post on social media platform X, stating the next launch attempt of the spacecraft could take place in about four weeks.
The comment was posted on Musk’s official account on social media platform X.
Musk’s update suggests that Starship Flight 12 could target a launch around early April, though the schedule will depend on several remaining milestones at SpaceX’s Starbase launch facility in Texas.
Among the key steps is testing and certification of the site’s new launch tower, launch mount, and tank farm systems. These upgrades will support the next generation of Starship vehicles.
Booster 19 is expected to roll to the launch site and be placed on the launch mount before returning to the production facility to receive its 33 Raptor engines. The booster would then return for a static fire test, which could mark the first time a Super Heavy booster equipped with Raptor V3 engines is fired on the pad.
Ship 39 is expected to undergo a similar preparation process. The vehicle will likely return to the production site to receive its six engines before heading to Massey’s test site for static fire testing.
Once both stages are prepared, the booster and ship will roll out to the launch site for the first full stack of a V3 Super Heavy and V3 Starship. A full wet dress rehearsal is expected to follow before any launch attempt.
Elon Musk has previously shared how SpaceX plans to eventually recover Starship’s upper stage using the launch tower’s robotic arms. Musk noted that the company will only attempt to catch the Starship spacecraft after two successful soft landings in the ocean. The approach is intended to reduce risk before attempting a recovery over land.
“Should note that SpaceX will only try to catch the ship with the tower after two perfect soft landings in the ocean. The risk of the ship breaking up over land needs to be very low,” Musk wrote in a post on X.
Such a milestone would represent a major step toward the full reuse of the Starship system, which remains a central goal for SpaceX’s long-term launch strategy.








