Elon Musk appeared for a short but productive interview at the Wall Street Journal’s (WSJ) CEO Council Summit on Tuesday, December 7. Musk provided updates on each of his companies’ most interesting projects, including Tesla’s Cybertruck, SpaceX’s Starship, and Neuralink’s brain implant.
The CEO of many forward-thinking companies seemed very optimistic about Neuralink in particular and its potential in the future. At the summit, Musk stated that Neuralink hopes to start human trials in 2022, pending FDA approval.
“We hope to have this in our first humans, which will be people who have severe spinal cord injuries like tetraplegics, quadriplegics next year pending FDA approval. And, I should say, our standards for implanting the device are substantially higher than what the FDA requires. Just as our standards for safety with Tesla are much higher than what the US government requires,” the Neuralink CEO said.
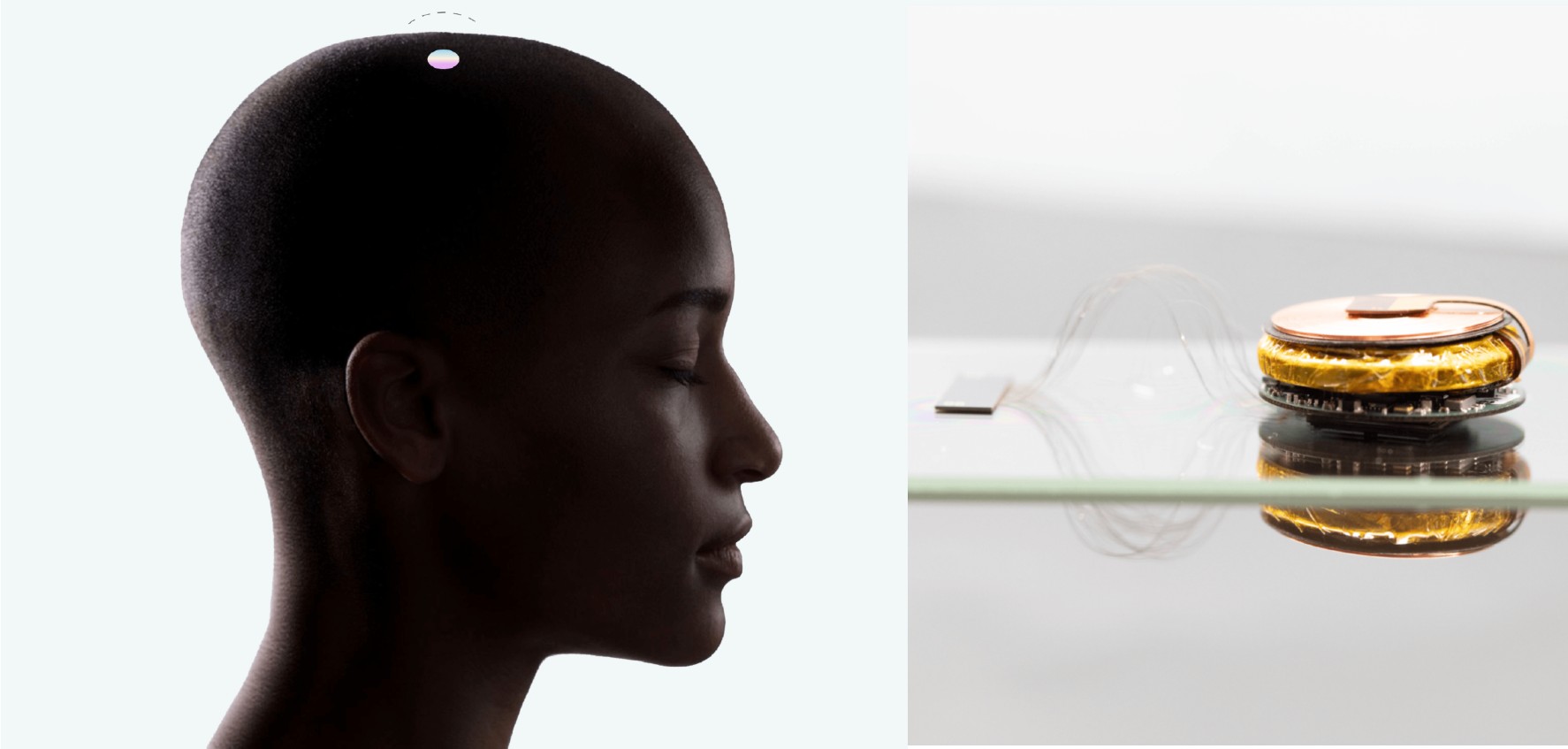
Tetraplegia, also known as quadriplegia, is a form of paralysis that affects both arms and legs. Tetraplegia-causing injury can vary in severity. Neuralink has created a device called the N1 Link, intended to help patients like those who are tetraplegic.
The N1 Link is a 1024 channel device that Neuralink made for patients’ therapeutic use. Once the device is implanted into a patient’s brain, it is expected to invisibly transmit data via a wireless connection. Neuralink demoed the outcome of a successfully implanted N1 Link in its video featuring a Macaque monkey named Pager, playing Pong with his mind.
Elon Musk is cautiously optimistic about the N1 Link’s future. He believes Neuralink’s device could help people with severe spinal cord injuries.
“I think it’s something pretty cool, and I do want to say that I’m very, emphasis on cautiously, optimistic about this. I think we have a chance with Neuralink of being able to restore full body functionality to someone who has a spinal cord injury.
“Meaning, I think we have a chance—I emphasize a chance—of being able to allow someone who cannot walk or use their arms to be able to walk again naturally. It’s a super big deal, and I don’t want to raise hopes unreasonably. But I’m increasingly convinced that this can be done,” Musk said.
For now, Neuralink is focusing on the N1 Link’s potential to help quadriplegics regain their digital freedom by giving them the ability to interact with their computers or phones in a high bandwidth and naturalistic way. In July, the company announced a Series C funding round of $205 million, which will take the N1 Link to market. Part of the funds will also accelerate research and development in other products.
Elon Musk’s short-term goal for Neuralink is to help people with brain injuries. Neuralink’s long-term goal is to reduce AI risk to humanity.
Watch Elon Musk’s WSJ interview below!
The Teslarati team would appreciate hearing from you. If you have any tips, reach out to me at maria@teslarati.com or via Twitter @Writer_01001101.

News
Tesla preps to build its most massive Supercharger yet: 400+ V4 stalls
The project will be an expansion of the current Eddie World Supercharger in Yermo, California, and will take place in several stages.

Tesla is preparing to build its most massive Supercharger yet, as it recently submitted plans for an over 400-stall Supercharging station in California, which would dwarf its massive 168-stall location in Lost Hills, California.
The project will be an expansion of the current Eddie World Supercharger in Yermo, California, and will take place in several stages.
The expansion, adjacent to the existing Eddie World Supercharger, which is currently comprised of 22 older V2 and V3 stalls limited to 150 kW, unfolds across six phases.
Construction on Phase 1 begins later this year with 72 V4 stalls. Subsequent stages will progressively add hundreds more, culminating in over 400 next-generation chargers. Site plans label expansive parking arrays across Phases 1–5 along Calico Boulevard, with Phase 6 design still to be determined.
Tesla is planning an absolutely massive Supercharger expansion in Yermo, California!!
Over the course of 6 phases, Tesla is set to add over 400 V4 stalls in a commercial development known as Eddie World 2.
The first phase, which should begin construction sometime this year,… pic.twitter.com/ks5Y5dE8lR
— MarcoRP (@MarcoRPi1) March 6, 2026
The project was first flagged by MarcoRP, a notable Tesla Supercharger watcher.
Strategically located midway on I-15 between Los Angeles and Las Vegas, the station targets heavy EV traffic on this high-demand corridor.
The surrounding 20-mile stretch already hosts over 200 high-power stalls (including 40 at 250 kW, 120 at 325 kW, and more), plus 96 in nearby Baker—yet bottlenecks persist during peak travel.
In scale, it eclipses all existing Tesla Superchargers. The current record holder, the solar- and Megapack-powered “Project Oasis” in Lost Hills, California, offers 164 stalls. Barstow’s former leader had 120. Eddie World 2 will be more than double that size, cementing Tesla’s dominance in ultra-high-capacity charging.
Tesla finishes its biggest Supercharger ever with 168 stalls
Development blends charging with convenience. Architectural drawings show integrated retail: a 10,100 square foot Cracker Barrel, a 4,300 square foot McDonald’s, a 3,800 square foot convenience store, additional restaurants, drive-thrus, outdoor dining, and lease space.
EV-centric features include pull-through bays for Cybertrucks and trailers, ensuring accessibility for larger vehicles and future Semi trucks.
News
Tesla makes latest move to remove Model S and Model X from its lineup
Tesla’s latest decisive step toward phasing out its flagship sedan and SUV was quietly removing the Model S and Model X from its U.S. referral program earlier this week.

Tesla has made its latest move that indicates the Model S and Model X are being removed from the company’s lineup, an action that was confirmed by the company earlier this quarter, that the two flagship vehicles would no longer be produced.
Tesla has ultimately started phasing out the Model S and Model X in several ways, as it recently indicated it had sold out of a paint color for the two vehicles.
Now, the company is making even more moves that show its plans for the two vehicles are being eliminated slowly but surely.
Tesla’s latest decisive step toward phasing out its flagship sedan and SUV was quietly removing the Model S and Model X from its U.S. referral program earlier this week.
The change eliminates the $1,000 referral discount previously available to new buyers of these vehicles. Existing Tesla owners purchasing a new Model S or Model X will now only receive a halved loyalty discount of $500, down from $1,000.
The updates extend beyond the two flagship vehicles. New Cybertruck buyers using a referral code on Premium AWD or Cyberbeast configurations will no longer get $1,000 off. Instead, both referrer and buyer receive three months of Full Self-Driving (Supervised).
The loyalty discount for Cybertruck purchases, excluding the new Dual Motor AWD trim level, has also been cut to $500.
NEWS: Tesla has removed the Model S and Model X from the referral program.
New owners also no longer get a $1,000 referral discount on a new Cybertruck Premium AWD or Cyberbeast. Instead, you now get 3 months of FSD (Supervised).
Additionally, Tesla has reduced the loyalty… pic.twitter.com/IgIY8Hi2WJ
— Sawyer Merritt (@SawyerMerritt) March 6, 2026
These adjustments apply only in the United States, and reflect Tesla’s broader strategy to optimize margins while boosting adoption of its autonomous driving software.
The timing is no coincidence. Tesla confirmed earlier this year that Model S and Model X production will end in the second quarter of 2026, roughly June, as the company reallocates factory capacity toward its Optimus humanoid robot and next-generation vehicles.
With annual sales of the low-volume flagships already declining (just 53,900 units in 2025), incentives are no longer needed to drive demand. Production is winding down, and Tesla expects strong remaining interest without subsidies.
Industry observers see this as the clearest sign yet of an “end-of-life” phase for the vehicles that once defined Tesla’s luxury segment. Community reactions on X range from nostalgia, “Rest in power S and X”, to frustration among long-time owners who feel perks are eroding just as the models approach discontinuation.
Some buyers are rushing orders to lock in final discounts before they vanish entirely.
Doug DeMuro names Tesla Model S the Most Important Car of the last 30 years
For Tesla, the move prioritizes efficiency: fewer discounts on outgoing models, a stronger push for FSD subscriptions, and a focus on high-margin Cybertruck trims amid surging orders.
Loyalists still have a narrow window to purchase a refreshed Plaid or Long Range model with remaining incentives, but the message is clear: Tesla’s lineup is evolving, and the era of the original flagships is drawing to a close.
News
Tesla Australia confirms six-seat Model Y L launch in 2026
Compared with the standard five-seat Model Y, the Model Y L features a longer body and extended wheelbase to accommodate an additional row of seating.

Tesla has confirmed that the larger six-seat Model Y L will launch in Australia and New Zealand in 2026.
The confirmation was shared by techAU through a media release from Tesla Australia and New Zealand.
The Model Y L expands the Model Y lineup by offering additional seating capacity for customers seeking a larger electric SUV. Compared with the standard five-seat Model Y, the Model Y L features a longer body and extended wheelbase to accommodate an additional row of seating.
The Model Y L is already being produced at Tesla’s Gigafactory Shanghai for the Chinese market, though the vehicle will be manufactured in right-hand-drive configuration for markets such as Australia and New Zealand.
Tesla Australia and New Zealand confirmed the vehicle will feature seating for six passengers.
“As shown in pictures from its launch in China, Model Y L will have a new seating configuration providing room for 6 occupants,” Tesla Australia and New Zealand said in comments shared with techAU.
Instead of a traditional seven-seat arrangement, the Model Y L uses a 2-2-2 layout. The middle row features two individual seats, allowing easier access to the third row while providing additional space for passengers.
Tesla Australia and New Zealand also confirmed that the Model Y L will be covered by the company’s updated warranty structure beginning in 2026.
“As with all new Tesla Vehicles from the start of 2026, the Model Y L will come with a 5-year unlimited km vehicle warranty and 8 years for the battery,” the company said.
The updated policy increases Tesla’s vehicle warranty from the previous four-year or 80,000-kilometer coverage.
Battery and drive unit warranties remain unchanged depending on the variant. Rear-wheel-drive models carry an eight-year or 160,000-kilometer warranty, while Long Range and Performance variants are covered for eight years or 192,000 kilometers.
Tesla has not yet announced official pricing or range figures for the Model Y L in Australia.