News
Elon Musk’s Neuralink seeking partner for clinical trials: Reuters
Elon Musk’s brain-machine company, Neuralink, has reportedly approached Barrow Neurological Institute, one of the premier neurosurgery centers in the United States, as a potential partner for clinical trials. The information is reportedly shared by people familiar with the matter.
The sources, who requested to be anonymous, told Reuters that Neuralink has been talking with Barrow to help it with its human trials. The sources, however, noted that the talks might not lead to a partnership. Neuralink is reportedly exploring a potential collaboration with other centers as well.
Neuralink has been working on brain implants since 2016, with the goal of treating conditions like paralysis and blindness. Elon Musk has been quite optimistic about Neuralink’s potential, and the company has successfully implanted its devices in animals. The company’s technology is yet to be tested on humans, however.
As per previous reports, Neuralink reportedly faced a setback when the US Food and Drug Administration (FDA) allegedly rejected its application for human trials, citing safety concerns. The company has been working to address these concerns, but it remains uncertain when or if it will succeed, the publication noted.
Neuralink representatives have not issued a comment about the matter, and the same was true for the FDA. Interestingly enough, Francisco Ponce, director of Barrow’s Center for Neuromodulation and Neurosurgery Residency Program, noted that Barrow is well-positioned to conduct implant research due to its extensive experience in the field.
Barrow has contributed to the standardization of brain implant surgeries in which patients can remain asleep during the procedure. This, Ponce noted, was a critical step in making the procedure more broadly acceptable. In a way, this aligns with Musk’s vision for Neuralink’s brain chip, as the Tesla CEO aims to make brain implants as common as Lasik eye surgery in the future.
It should be noted that Barrow is well experienced in deep brain stimulation devices, which have been FDA-approved since 1997. In contrast, Neuralink’s implant is a brain-computer interface (BCI) device that uses electrodes to either penetrate the brain or rest on its surface, facilitating direct communication with devices like computers.
Don’t hesitate to contact us with news tips. Just send a message to simon@teslarati.com to give us a heads up.
Elon Musk
Tesla Supercharger Diner food menu gets a sneak peek as construction closes out
What are you ordering at the Tesla Diner?

The Tesla Supercharger Diner in Los Angeles is nearing completion as construction appears to be winding down significantly. However, the more minor details, such as what the company will serve at its 50s-style diner for food, are starting to be revealed.
Tesla’s Supercharger Diner is set to open soon, seven years after CEO Elon Musk first drafted the idea in a post on X in 2018. Musk has largely come through on most of what he envisioned for the project: the diner, the massive movie screens, and the intended vibe are all present, thanks to the aerial and ground footage shared on social media.
We already know the Diner will be open 24/7, based on decals placed on the front door of the restaurant that were shared earlier this week. We assume that Tesla Optimus will come into play for these long and uninterrupted hours.
The Tesla Diner is basically finished—here’s what it looks like
As far as the food, Tesla does have an email also printed on the front door of the Diner, but we did not receive any response back (yet) about what cuisine it will be offering. We figured it would be nothing fancy and it would be typical diner staples: burgers, fries, wings, milkshakes, etc.
According to pictures taken by @Tesla_lighting_, which were shared by Not a Tesla App, the food will be just that: quick and affordable meals that diners do well. It’s nothing crazy, just typical staples you’d find at any diner, just with a Tesla twist:
Tesla Diner food:
• Burgers
• Fries
• Chicken Wings
• Hot Dogs
• Hand-spun milkshakes
• And more https://t.co/kzFf20YZQq pic.twitter.com/aRv02TzouY— Sawyer Merritt (@SawyerMerritt) July 17, 2025
As the food menu is finalized, we will be sure to share any details Tesla provides, including a full list of what will be served and its prices.
Additionally, the entire property appears to be nearing its final construction stages, and it seems it may even be nearing completion. The movie screens are already up and showing videos of things like SpaceX launches.
There are many cars already using the Superchargers at the restaurant, and employees inside the facility look to be putting the finishing touches on the interior.
🚨 Boots on the ground at the Tesla Diner:
— TESLARATI (@Teslarati) July 17, 2025
It’s almost reminiscent of a Tesla version of a Buc-ee’s, a southern staple convenience store that offers much more than a traditional gas station. Of course, Tesla’s version is futuristic and more catered to the company’s image, but the idea is the same.
It’s a one-stop shop for anything you’d need to recharge as a Tesla owner. Los Angeles building permits have not yet revealed the date for the restaurant’s initial operation, but Tesla may have its eye on a target date that will likely be announced during next week’s Earnings Call.
News
Tesla’s longer Model Y did not scale back requests for this vehicle type from fans
Tesla fans are happy with the new Model Y, but they’re still vocal about the need for something else.

Tesla launched a slightly longer version of the Model Y all-electric crossover in China, and with it being extremely likely that the vehicle will make its way to other markets, including the United States, fans are still looking for something more.
The new Model Y L in China boasts a slightly larger wheelbase than its original version, giving slightly more interior room with a sixth seat, thanks to a third row.
Tesla exec hints at useful and potentially killer Model Y L feature
Tesla has said throughout the past year that it would focus on developing its affordable, compact models, which were set to begin production in the first half of the year. The company has not indicated whether it met that timeline or not, but many are hoping to see unveilings of those designs potentially during the Q3 earnings call.
However, the modifications to the Model Y, which have not yet been officially announced for any markets outside of China, still don’t seem to be what owners and fans are looking forward to. Instead, they are hoping for something larger.
A few months ago, I reported on the overall consensus within the Tesla community that the company needs a full-size SUV, minivan, or even a cargo van that would be ideal for camping or business use.
Tesla is missing one type of vehicle in its lineup and fans want it fast
That mentality still seems very present amongst fans and owners, who state that a full-size SUV with enough seating for a larger family, more capability in terms of cargo space for camping or business operation, and something to compete with gas cars like the Chevrolet Tahoe, Ford Expedition, or electric ones like the Volkswagen ID.BUZZ.
We asked the question on X, and Tesla fans were nearly unanimously in support of a larger SUV or minivan-type vehicle for the company’s lineup:
🚨 More and more people are *still* saying that, despite this new, longer Model Y, Tesla still needs a true three-row SUV
Do you agree? https://t.co/QmbRDcCE08 pic.twitter.com/p6m5zB4sDZ
— TESLARATI (@Teslarati) July 16, 2025
Here’s what some of the respondents said:
100% agree, we need a larger vehicle.
Our model Y is quickly getting too small for our family of 5 as the kids grow. A slightly longer Y with an extra seat is nice but it’s not enough if you’re looking to take it on road trips/vacations/ kids sports gear etc.
Unfortunately we…
— Anthony Hunter (@_LiarsDice_) July 17, 2025
Had to buy a Kia Carnival Hybrid because Tesla doesn’t have a true 3 row vehicle with proper space and respectable range. pic.twitter.com/pzwFyHU8Gi
— Neil, like the astronaut (@Neileeyo) July 17, 2025
Agreed! I’m not sure who created this but I liked it enough to save it. pic.twitter.com/Sof5nMehjS
— 🦉Wise Words of Wisdom – Inspirational Quotes (IQ) (@WiseWordsIQ) July 16, 2025
Tesla is certainly aware that many of its owners would like the company to develop something larger that competes with the large SUVs on the market.
However, it has not stated that anything like that is in the current plans for future vehicles, as it has made a concerted effort to develop Robotaxi alongside the affordable, compact models that it claims are in development.
It has already unveiled the Robovan, a people-mover that can seat up to 20 passengers in a lounge-like interior.
The Robovan will be completely driverless, so it’s unlikely we will see it before the release of a fully autonomous Full Self-Driving suite from Tesla.
Energy
Tesla launches first Virtual Power Plant in UK – get paid to use solar
Tesla has launched its first-ever Virtual Power Plant program in the United Kingdom.
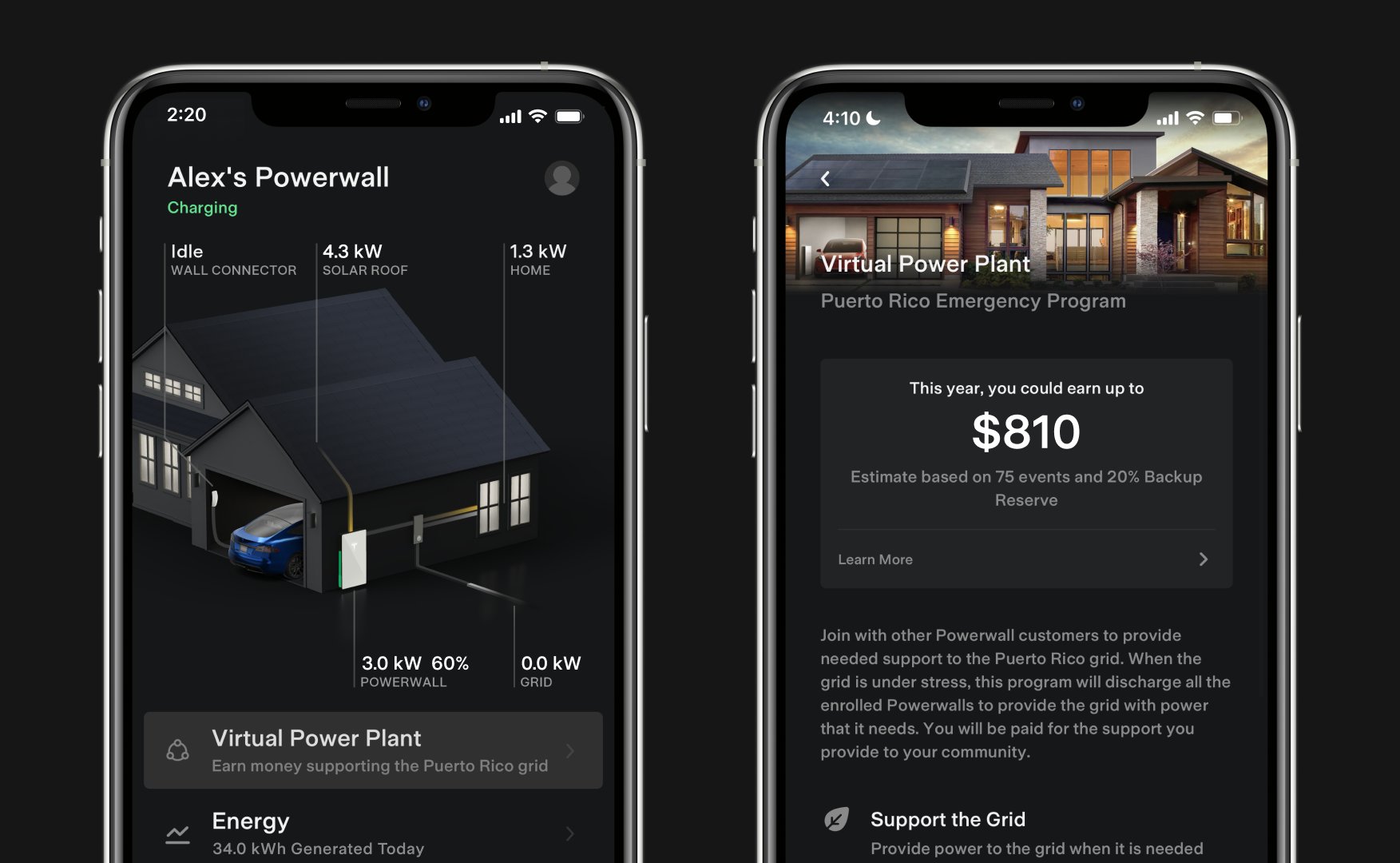
Tesla has launched its first-ever Virtual Power Plant program in the United Kingdom. This feature enables users of solar panels and energy storage systems to sell their excess energy back to the grid.
Tesla is utilizing Octopus Energy, a British renewable energy company that operates in multiple markets, including the UK, France, Germany, Italy, Spain, Australia, Japan, New Zealand, and the United States, as the provider for the VPP launch in the region.
The company states that those who enroll in the program can earn up to £300 per month.
Tesla has operated several VPP programs worldwide, most notably in California, Texas, Connecticut, and the U.S. territory of Puerto Rico. This is not the first time Tesla has operated a VPP outside the United States, as there are programs in Australia, Japan, and New Zealand.
This is its first in the UK:
Our first VPP in the UK
You can get paid to share your energy – store excess energy in your Powerwall & sell it back to the grid
You’re making £££ and the community is powered by clean energy
Win-win pic.twitter.com/evhMtJpgy1
— Tesla UK (@tesla_uk) July 17, 2025
Tesla is not the only company that is working with Octopus Energy in the UK for the VPP, as it joins SolarEdge, GivEnergy, and Enphase as other companies that utilize the Octopus platform for their project operations.
It has been six years since Tesla launched its first VPP, as it started its first in Australia back in 2019. In 2024, Tesla paid out over $10 million to those participating in the program.
Participating in the VPP program that Tesla offers not only provides enrolled individuals with the opportunity to earn money, but it also contributes to grid stabilization by supporting local energy grids.
-

 Elon Musk1 day ago
Elon Musk1 day agoWaymo responds to Tesla’s Robotaxi expansion in Austin with bold statement
-

 News1 day ago
News1 day agoTesla exec hints at useful and potentially killer Model Y L feature
-

 Elon Musk2 days ago
Elon Musk2 days agoElon Musk reveals SpaceX’s target for Starship’s 10th launch
-

 Elon Musk3 days ago
Elon Musk3 days agoTesla ups Robotaxi fare price to another comical figure with service area expansion
-

 News1 day ago
News1 day agoTesla’s longer Model Y did not scale back requests for this vehicle type from fans
-

 News1 day ago
News1 day ago“Worthy of respect:” Six-seat Model Y L acknowledged by Tesla China’s biggest rivals
-

 News2 days ago
News2 days agoFirst glimpse of Tesla Model Y with six seats and extended wheelbase
-
 Elon Musk2 days ago
Elon Musk2 days agoElon Musk confirms Tesla is already rolling out a new feature for in-car Grok