Neuralink has responded to claims of inhumane treatment during the animal testing phases of various company products, stating that animal welfare is a priority. “At Neuralink, we are absolutely committed to working with animals in the most humane and ethical way possible.”
Last week, reports surfaced of a lawsuit against the University of California at Davis from the Physicians Committee of Responsible Medicine (PCRM). The suit claims that the facility “failed to provide dying monkeys with adequate veterinary care, used an unapproved substance known as “Bioglue” that killed monkeys by destroying portions of their brains, and failed to provide for the psychological well-being of monkeys assigned to the experiment.” Earlier today, Teslarati reported an extensive timeline of events from the beginning of the partnership between UC Davis and Neuralink to the most recent developments, which include the potential of videos and photographs of the animals involved in the testing. Teslarati obtained several copies of veterinarian records from autopsies of some monkeys used in the experiments.
Neuralink is now responding to the various claims of animal abuse in a lengthy statement that outlines the past, present, and future developments of Neuralink trials. The company maintains that all animals are treated respectfully and ethically.
EXCLUSIVE: Neuralink dragged into humane testing lawsuit – Timeline of Events
Neuralink ended its partnership with UC Davis in November 2020, just two months after PCRM sent a California Public Records request for information regarding the Neuralink trials. The request is eventually denied in accordance with California State Code 6255(a), which says that the Agency “shall justify withholding any record by demonstrating that the record in question is exempt under express provisions of this chapter or that on the facts of the particular case the public interest served by not disclosing the record clearly outweighs the public interest served by disclosure of the record.”
Neuralink took several of the monkeys back to its testing facilities in Northern California for in-house experiments. “Once construction of our in-house facility was completed, we were able to bring some unimplanted macaques from UC Davis with us to Neuralink. This included Pager, who would later be implanted with our Neuralink device and go on to achieve outstanding brain-computer interface performance, while freely behaving and unrestrained, as demonstrated in the Monkey MindPong video,” the company’s official response said. “While the facilities and care at UC Davis did and continue to meet federally mandated standards, we absolutely wanted to improve upon these standards as we transitioned animals to our in-house facilities.”
Neuralink says that “no injuries occurred at any time to animals housed at UC Davis” while the tests were ongoing. The company admits several animals were euthanized for research purposes, but they were done under controlled circumstances:
“The initial work from these procedures allowed us to develop our novel surgical and robot procedures, establishing safer protocols for subsequent survival surgeries. Survival studies then allowed us to test the function of different generations of implanted devices as we refined them towards human use. The use of every animal was extensively planned and considered to balance scientific discovery with the ethical use of animals. As part of this work, two animals were euthanized at planned end dates to gather important histological data, and six animals were euthanized at the medical advice of the veterinary staff at UC Davis. These reasons included one surgical complication involving the use of the FDA-approved product (BioGlue), one device failure, and four suspected device-associated infections, a risk inherent with any percutaneous medical device. In response we developed new surgical protocols and a fully implanted device design for future surgeries.”
Interestingly, PCRM said in a press release that “BioGlue” is an unapproved substance. FDA documents obtained by Teslarati show that BioGlue was approved for use in 2001, but the agency also included a warning of potential side effects when BioGlue is applied to the phrenic nerve. Application of BioGlue to this area in animals can cause acute nerve injury. Additionally, “BioGlue application to the surface of the heart can cause coagulation necrosis that extends into the myocardium, which could reach underlying conduction tissue and may cause acute, focal sinoatrial node degeneration,” the documents said. Five pigs were tested during initial animal experimentation while the FDA was determining BioGlue’s safety. All five pigs survived to the designated observation time.
Neuralink details humane animal treatment during Link v0.9 testing
Presently, the animals involved in Neuralink testing are housed at the company’s 6,000-square-foot facility that houses farm animals and rhesus macaques. The company takes care of the animals from the time they enter the facility to the time they leave, even detailing an animal’s process for “retirement:”
“Can we release the animals that regularly choose not to participate or who have completed their contribution to the study? Yes! We opted to retire animals at the conclusion of their projects. We retired several macaques to a sanctuary last March because they consistently chose to spend their day swimming in their pools, foraging, and relaxing in their hammocks rather than attending the game we presented to them. Their brand new enclosures and sanctuary costs were fully funded by Neuralink.”
Moving forward, Neuralink says it is always working to improve the current standards for animal well-being. “We also look forward to a day where animals are no longer necessary for medical research. Yet our society currently relies on medical breakthroughs to cure diseases, prevent the spread of viruses, and create technology that can change how people are able to interact with the world. However, if animals must be used in research in the meantime, their lives and experiences should be as vital and naturalistic as possible. We will always strive to surpass the industry standard and never stop asking ourselves: “Can we do better for the animals?”, and never forget it is a privilege to work with animals in research. It is our responsibility as caretakers to ensure that their experience is as peaceful and frankly, as joyful as possible.”
Neuralink’s complete statement is available here.
I’d love to hear from you! If you have any comments, concerns, or questions, please email me at joey@teslarati.com. You can also reach me on Twitter @KlenderJoey, or if you have news tips, you can email us at tips@teslarati.com.
News
Tesla UK sales see 14% year-over-year rebound in June: SMMT data
The SMMT stated that Tesla sales grew 14% year-over-year to 7,719 units in June 2025.

Tesla’s sales in the United Kingdom rose in June, climbing 14% year-over-year to 7,719 units, as per data from the Society of Motor Manufacturers and Traders (SMMT). The spike in the company’s sales coincided with the first deliveries of the updated Model Y last month.
Model Y deliveries support Tesla’s UK recovery
Tesla’s June performance marked one of its strongest months in the UK so far this year, with new Model Y deliveries contributing significantly to the company’s momentum.
While the SMMT listed Tesla with 7,719 deliveries in June, independent data from New AutoMotive suggested that the electric vehicle maker registered 7,891 units during the month instead. However, year-to-date figures for Tesla remain 2% down compared to 2024, as per a report from Reuters.
While Tesla made a strong showing in June, rivals are also growing. Chinese automaker BYD saw UK sales rise nearly fourfold to 2,498 units, while Ford posted the highest EV growth among major automakers, with a more than fourfold increase in the first half of 2025.
Overall, the UK’s battery electric vehicle (BEV) demand surged 39% to to 47,354 units last month, helping push total new car sales in the UK to 191,316 units, up 6.7% from the same period in 2024.
EV adoption accelerates, but concerns linger
June marked the best month for UK car sales since 2019, though the SMMT cautioned that growth in the electric vehicle sector remains heavily dependent on discounting and support programs. Still, one in four new vehicle buyers in June chose a battery electric vehicle.
SMMT Chief Executive Mike Hawes noted that despite strong BEV demand, sales levels are still below regulatory targets. “Further growth in sales, and the sector will rely on increased and improved charging facilities to boost mainstream electric vehicle adoption,” Hawes stated.
Also taking effect this week was a new US-UK trade deal, which lowers tariffs on UK car exports to the United States from 27.5% to 10%. The agreement could benefit UK-based EV producers aiming to expand across the country.
News
Tesla Model 3 ranks as the safest new car in Europe for 2025, per Euro NCAP tests
Despite being on the market longer than many of its rivals, the Tesla Model 3 continues to set the bar for vehicle safety.

The Tesla Model 3 has been named the safest new car on sale in 2025, according to the latest results from the Euro NCAP. Among 20 newly tested vehicles, the Model 3 emerged at the top of the list, scoring an impressive 359 out of 400 possible points across all major safety categories.
Tesla Model 3’s safety systems
Despite being on the market longer than many of its rivals, the Tesla Model 3 continues to set the bar for vehicle safety. Under Euro NCAP’s stricter 2025 testing protocols, the electric sedan earned 90% for adult occupant protection, 93% for child occupant protection, 89% for pedestrian protection, and 87% for its Safety Assist systems.
The updated Model 3 received particular praise for its advanced driver assistance features, including Tesla’s autonomous emergency braking (AEB) system, which performed well across various test scenarios. Its Intelligent Speed Assistance and child presence detection system were cited as noteworthy features as well, as per a WhatCar report.
Other notable safety features include the Model 3’s pedestrian-friendly pop-up hood and robust crash protection for both front and side collisions. Euro NCAP also highlighted the Model 3’s ability to detect vulnerable road users during complex maneuvers, such as turning across oncoming traffic.
Euro NCAP’s Autopilot caution
While the Model 3’s safety scores were impressive across the board, Euro NCAP did raise concerns about driver expectations of Tesla’s Autopilot system. The organization warned that some owners may overestimate the system’s capabilities, potentially leading to misuse or inattention behind the wheel. Even so, the Model 3 remained the highest-scoring vehicle tested under Euro NCAP’s updated criteria this year.
The Euro NCAP’s concerns are also quite interesting because Tesla’s Full Self-Driving (FSD) Supervised, which is arguably the company’s most robust safety suite, is not allowed for public rollout in Europe yet. FSD Supervised would allow the Model 3 to navigate inner city streets with only minimal human supervision.
Other top scorers included the Volkswagen ID.7, Polestar 3, and Geely EX5, but none matched the Model 3’s total score or consistency across categories. A total of 14 out of 20 newly tested cars earned five stars, while several models, including the Kia EV3, MG ZS, and Renault 5, fell short of the top rating.
Elon Musk
Why Tesla’s Q3 could be one of its biggest quarters in history
Tesla could stand to benefit from the removal of the $7,500 EV tax credit at the end of Q3.

Tesla has gotten off to a slow start in 2025, as the first half of the year has not been one to remember from a delivery perspective.
However, Q3 could end up being one of the best the company has had in history, with the United States potentially being a major contributor to what might reverse a slow start to the year.
Earlier today, the United States’ House of Representatives officially passed President Trump’s “Big Beautiful Bill,” after it made its way through the Senate earlier this week. The bill will head to President Trump, as he looks to sign it before his July 4 deadline.
The Bill will effectively bring closure to the $7,500 EV tax credit, which will end on September 30, 2025. This means, over the next three months in the United States, those who are looking to buy an EV will have their last chance to take advantage of the credit. EVs will then be, for most people, $7,500 more expensive, in essence.
The tax credit is available to any single filer who makes under $150,000 per year, $225,000 a year to a head of household, and $300,000 to couples filing jointly.
Ending the tax credit was expected with the Trump administration, as his policies have leaned significantly toward reliance on fossil fuels, ending what he calls an “EV mandate.” He has used this phrase several times in disagreements with Tesla CEO Elon Musk.
Nevertheless, those who have been on the fence about buying a Tesla, or any EV, for that matter, will have some decisions to make in the next three months. While all companies will stand to benefit from this time crunch, Tesla could be the true winner because of its sheer volume.
If things are done correctly, meaning if Tesla can also offer incentives like 0% APR, special pricing on leasing or financing, or other advantages (like free Red, White, and Blue for a short period of time in celebration of Independence Day), it could see some real volume in sales this quarter.
You can now buy a Tesla in Red, White, and Blue for free until July 14 https://t.co/iAwhaRFOH0
— TESLARATI (@Teslarati) July 3, 2025
Tesla is just a shade under 721,000 deliveries for the year, so it’s on pace for roughly 1.4 million for 2025. This would be a decrease from the 1.8 million cars it delivered in each of the last two years. Traditionally, the second half of the year has produced Tesla’s strongest quarters. Its top three quarters in terms of deliveries are Q4 2024 with 495,570 vehicles, Q4 2023 with 484,507 vehicles, and Q3 2024 with 462,890 vehicles.
-

 Elon Musk4 days ago
Elon Musk4 days agoTesla investors will be shocked by Jim Cramer’s latest assessment
-

 News1 week ago
News1 week agoTesla Robotaxi’s biggest challenge seems to be this one thing
-
 Elon Musk2 weeks ago
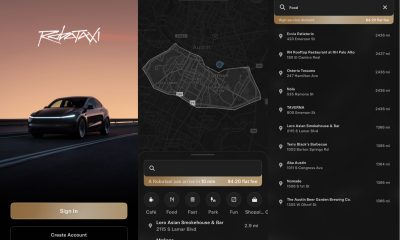
Elon Musk2 weeks agoFirst Look at Tesla’s Robotaxi App: features, design, and more
-

 News2 weeks ago
News2 weeks agoSpaceX and Elon Musk share insights on Starship Ship 36’s RUD
-

 News2 weeks ago
News2 weeks agoWatch Tesla’s first driverless public Robotaxi rides in Texas
-

 News1 week ago
News1 week agoWatch the first true Tesla Robotaxi intervention by safety monitor
-

 News2 weeks ago
News2 weeks agoTesla has started rolling out initial round of Robotaxi invites
-

 Elon Musk2 weeks ago
Elon Musk2 weeks agoTesla to launch in India in July with vehicles already arriving: report


















